Respiratory physician Lutz Beckert considers chronic obstructive pulmonary disease management, including the prevention of COPD, the importance of smoking cessation and pulmonary rehabilitation, and the lifesaving potential of addressing treatable traits. He also discusses the logic of inhaler therapy, moving from single therapy to dual and triple therapy when indicated, as well as other aspects of management
Shingles protection for adults with immunocompromise
+Summer Hiatus
In print
Practice + Vaccines
Shingles protection for adults with immunocompromise
Thursday 9 January 2025, 12:30 PM
We are on our summer break and the editorial office is closed until 13 January. In the meantime, please enjoy our Summer Hiatus series, in which our journalists curate an eclectic mix from our news and clinical archives throughout the year, The Conversation and other publications we share content with. Please note the comment function has been turned off while we are away. Happy reading!
This article was first published online on 25 September 2024.
This article details the recent widening of eligibility for funded herpes zoster vaccine to immunocompromised adults
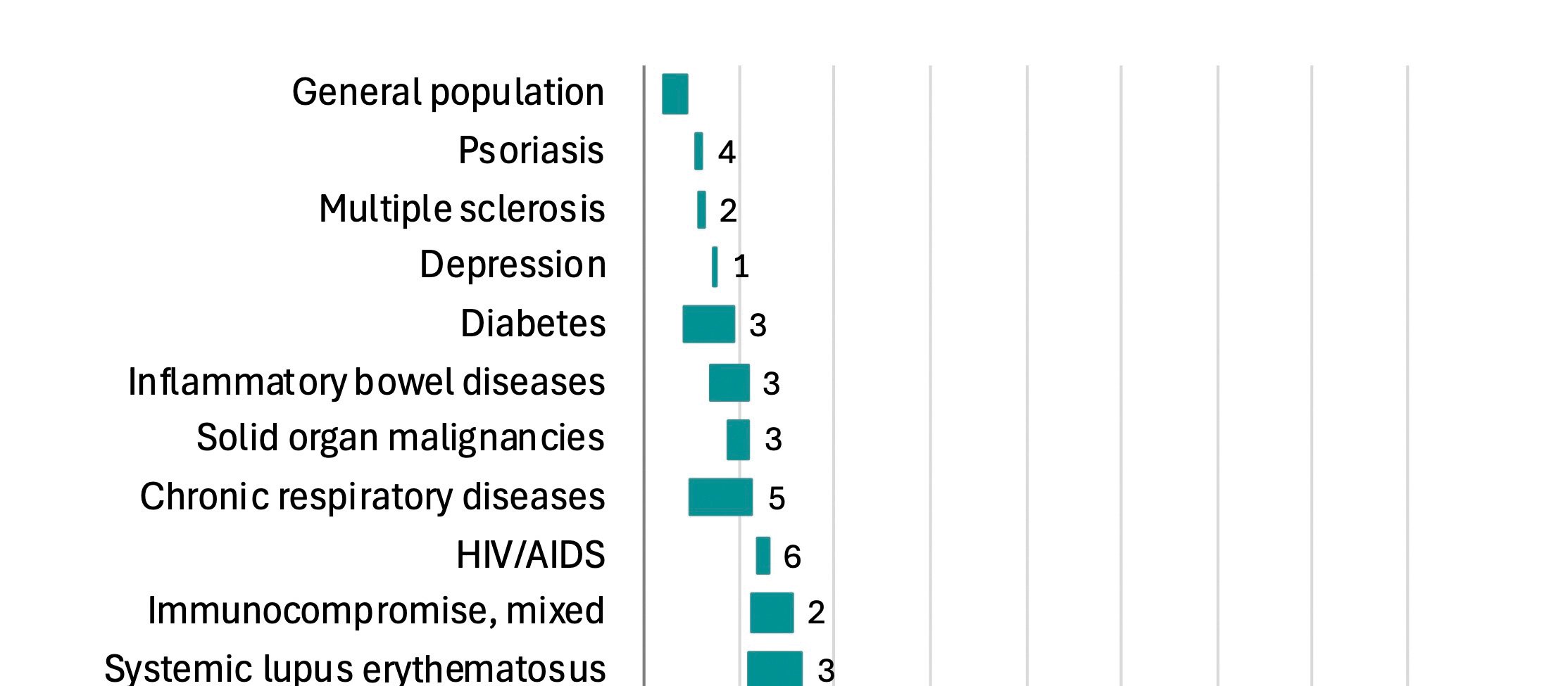
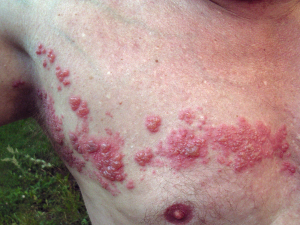
Key points, Herpes zoster is a painful and potentially severe condition.
Highest incidence of zoster is seen in those with immunocompromise and/or older age.
, Pract Green w Pale Yellow
Kia ora and welcome to New Zealand Doctor Rata Aotearoa
Not a subscriber? Unlock this article by subscribing here.
References
- Bhavsar A, Lonnet G, Wang C, et al. Increased risk of herpes zoster in adults ≥50 years old diagnosed with COVID-19 in the United States. Open Forum Infect Dis 2022;9(5):ofac118.
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. NEJM 2016;375(11):1019–32.
- Goud R, Lufkin B, Duffy J, et al. Risk of Guillain-Barre syndrome following recombinant zoster vaccine in Medicare beneficiaries. JAMA Intern Med 2021;181(12):1623–30.
- Izurieta HS, Wu X, Forshee R, et al. Recombinant zoster vaccine (Shingrix): Real-world effectiveness in the first 2 years post-licensure. Clin Infect Dis 2021;73(6):941–48.
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. NEJM 2015;372(22):2087–96.
- Marijam A, Vroom N, Bhavsar A, et al. Systematic literature review on the incidence of herpes zoster in populations at increased risk of disease in the EU/EEA, Switzerland, and the UK. Infect Dis Ther 2024;13(5):1083–104.
- Oostvogels L, Heineman TC, Johnson RW, et al. Medical conditions at enrolment do not impact efficacy and safety of the adjuvanted recombinant zoster vaccine: a pooled post-hoc analysis of two parallel randomized trials. Hum Vacc Immunother 2019;15(12):2865–72.
- Racine E, Gilca V, Amini R, et al. A systematic literature review of the recombinant subunit herpes zoster vaccine use in immunocompromised 18-49-year-old patients. Vaccine 2020;38(40):6205–14.
- Strezova A, Diez-Domingo J, Al Shawafi K, et al. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: Interim efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect Dis 2022;9(10):ofac485.
- Tavares-Da-Silva F, Co MM, Dessart C, et al. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine 2020;38(18):3489–500.
- Walia A, Sun Y, Acharya NR. Risk of herpes zoster ophthalmicus recurrence after recombinant zoster vaccination. JAMA Ophthalmol 2024;142(3):249–56.





![Barbara Fountain, editor of New Zealand Doctor Rata Aotearoa, and Paul Hutchison, GP and senior medical clinician at Tāmaki Health [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/Barbara%20Fountain%2C%20editor%20of%20New%20Zealand%20Doctor%20Rata%20Aotearoa%2C%20and%20Paul%20Hutchison%2C%20GP%20and%20senior%20medical%20clinician%20at%20T%C4%81maki%20Health%20CR%20Simon%20Maude.jpg?itok=-HbQ1EYA)
![Lori Peters, NP and advanced health improvement practitioner at Mahitahi Hauora, and Jasper Nacilla, NP at The Terrace Medical Centre in Wellington [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/2.%20Lori%20Peters%2C%20NP%20and%20advanced%20HIP%20at%20Mahitahi%20Hauora%2C%20and%20Jasper%20Nacilla%2C%20NP%20at%20The%20Terrace%20Medical%20Centre%20in%20Wellington%20CR%20Simon%20Maude.jpg?itok=sUfbsSF1)
![Ministry of Social Development health and disability coordinator Liz Williams, regional health advisors Mary Mojel and Larah Takarangi, and health and disability coordinators Rebecca Staunton and Myint Than Htut [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/3.%20Ministry%20of%20Social%20Development%27s%20Liz%20Williams%2C%20Mary%20Mojel%2C%20Larah%20Takarangi%2C%20Rebecca%20Staunton%20and%20Myint%20Than%20Htut%20CR%20Simon%20Maude.jpg?itok=9ceOujzC)
![Locum GP Helen Fisher, with Te Kuiti Medical Centre NP Bridget Woodney [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/4.%20Locum%20GP%20Helen%20Fisher%2C%20with%20Te%20Kuiti%20Medical%20Centre%20NP%20Bridget%20Woodney%20CR%20Simon%20Maude.jpg?itok=TJeODetm)
![Ruby Faulkner, GPEP2, with David Small, GPEP3 from The Doctors Greenmeadows in Napier [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/5.%20Ruby%20Faulkner%2C%20GPEP2%2C%20with%20David%20Small%2C%20GPEP3%20from%20The%20Doctors%20Greenmeadows%20in%20Napier%20CR%20Simon%20Maude.jpg?itok=B0u4wsIs)
![Rochelle Langton and Libby Thomas, marketing advisors at the Medical Protection Society [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/6.%20Rochelle%20Langton%20and%20Libby%20Thomas%2C%20marketing%20advisors%20at%20the%20Medical%20Protection%20Society%20CR%20Simon%20Maude.jpg?itok=r52_Cf74)
![Specialist GP Lucy Gibberd, medical advisor at MPS, and Zara Bolam, urgent-care specialist at The Nest Health Centre in Inglewood [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/7.%20Specialist%20GP%20Lucy%20Gibberd%2C%20medical%20advisor%20at%20MPS%2C%20and%20Zara%20Bolam%2C%20urgent-care%20specialist%20at%20The%20Nest%20Health%20Centre%20in%20Inglewood%20CR%20Simon%20Maude.jpg?itok=z8eVoBU3)
![Olivia Blackmore and Trudee Sharp, NPs at Gore Health Centre, and Gaylene Hastie, NP at Queenstown Medical Centre [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/8.%20Olivia%20Blackmore%20and%20Trudee%20Sharp%2C%20NPs%20at%20Gore%20Health%20Centre%2C%20and%20Gaylene%20Hastie%2C%20NP%20at%20Queenstown%20Medical%20Centre%20CR%20Simon%20Maude.jpg?itok=Z6u9d0XH)
![Mary Toloa, specialist GP at Porirua and Union Community Health Service in Wellington, Mara Coler, clinical pharmacist at Tū Ora Compass Health, and Bhavna Mistry, specialist GP at Porirua and Union Community Health Service [Image: Simon Maude]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-03/9.%20Mary%20Toloa%2C%20Porirua%20and%20Union%20Community%20Health%20Service%20in%20Wellington%2C%20Mara%20Coler%2C%20T%C5%AB%20Ora%20Compass%20Health%2C%20and%20Bhavna%20Mistry%2C%20PUCHS%20CR%20Simon%20Maude.jpg?itok=kpChr0cc)