Otolaryngologist, head and neck surgeon Francis T. Hall discusses the evaluation of thyroid nodules, which primarily aims to determine the likelihood of malignancy. He then reviews the treatment of thyroid nodules and thyroid cancer, including recent advances in management
Timely detection of herpes zoster ophthalmicus is key
Timely detection of herpes zoster ophthalmicus is key

This article discusses herpes zoster ophthalmicus and the importance of early diagnosis and prompt commencement of antiviral therapy
- Herpes zoster ophthalmicus is a common disease among older adults, with complications that carry a high risk of vision loss.
- Severe headache or V1 hyperalgesia may assist in the early diagnosis of HZO before development of rash.
- Ophthalmology referral is important to detect uveitis and keratitis.
- Both early commencement of antiviral therapy and vaccination are key to preventing serious complications.
This Practice article has been endorsed by the RNZCGP and has been approved for up to 0.25 credits for continuing professional development purposes (1 credit per learning hour). To claim your CPD credits, log in to your Te Whanake dashboard and record this activity under the appropriate learning category.
Nurses may also find that reading this article and reflecting on their learning can count as a professional development activity with the Nursing Council of New Zealand (up to 0.25 PD hours).
Shingles or herpes zoster is a common clinical presentation among older adults aged 60 and above.1,2 It results from reactivation of the varicella zoster virus, which is responsible for the common childhood disease, chickenpox. Following the primary chickenpox infection, the virus persists in dorsal ganglion cells of the adult nervous system before reactivating later in life as herpes zoster.3 As a result, painful vesicular rashes typically manifest along the involved dermatome.4
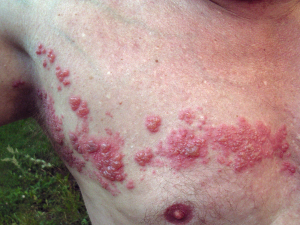
When the ophthalmic division (V1) of the trigeminal nerve along the face is involved during reactivation (Figures 1 and 2), this is called herpes zoster ophthalmicus.5 HZO accounts for 10–20 per cent of herpes zoster cases.6
The nasociliary nerve derives from the V1 division of the trigeminal nerve and innervates the anterior and posterior ethmoidal sinuses, skin of the eyelids and tip of the nose, conjunctiva, sclera, cornea, iris and the choroid. Vesicular rash involving the tip, side or root of the nose is associated with a higher rate of ocular involvement and is termed Hutchinson sign. Conjunctivitis, uveitis (iritis) and keratitis are the most common ocular manifestations of HZO.4,5
Prodromal symptoms of HZO may include fever, malaise, nausea and headache.5 Most patients with acute herpes zoster of the head experience pain prior to eruption of vesicles. Typically, the pain is of a stabbing quality, unilateral, moderate to severe and often wakes patients from sleep.7 Importantly, HZO must be considered by primary care physicians among older patients with V1 hyperalgesia not responding to simple analgesia, even in the absence of vesicular rash as the pain may sometimes precede the rash by several days.
Conjunctivitis appears to be the most common ocular complication of HZO, followed by keratitis (Figure 3) and anterior uveitis (Figure 4).4,8 Corneal scar, neurotrophic keratopathy, band keratopathy, corneal melt, corneal perforation and acute retinal necrosis or panuveitis are further complications of HZO that also contribute to vision loss.8
Vision loss (reduction in the best-corrected visual acuity by at least one line) has been reported in 12.4 per cent of patients after resolution of HZO.4 Permanent moderate vision loss (≤20/50) due to HZO was reported in 9.6 per cent of patients, and severe vision loss (≤20/200) in 3.6 per cent of patients, following an acute episode of HZO.8
Risk factors associated with vision loss include poor presenting visual acuity, older age, uveitis, immunosuppression and the number of HZO ocular manifestations. Older age is linked with uveitis and corneal involvement, which are also associated with vision loss after HZO.4,8
Prevention of corneal scarring from corneal involvement in HZO seems to be an important target for preventing vision loss. Patients with uveitis or keratitis often require prolonged topical corticosteroid for at least three months, and recurrence rates on corticosteroid cessation remain high.
Anterior uveitis is a common ocular complication of HZO that results in blurred vision and intense photophobia.4 It is marked by ciliary injection, as well as inflammatory cells and proteinaceous flare in the anterior chamber, and keratic precipitates on the cornea.9,10
Anterior uveitis usually develops within seven to 14 days of rash onset, and topical corticosteroid is required to control the anterior segment inflammation.8,9 Therefore, it is ideal to refer patients with HZO to an ophthalmologist seven to 14 days after onset of rash for consideration of topical corticosteroid therapy.
Earlier review is advised when there is blurring of vision or photophobia. Conjunctivitis or eyelid involvement alone does not necessitate early ophthalmology review.
Cranial nerve palsy and cerebrovascular accident, also known as stroke, are rare but important complications of HZO.
Cranial nerve palsy was reported in 3.5 per cent of patients in one study group, with cranial nerve III involvement being the most common, followed by cranial nerve VI.8 Double vision may result when patients experience external ocular motor palsies, but fortunately, most appear to be transient during acute HZO, resolving over a period of approximately three months.5
HZO has been reported to increase the risk of CVA when compared with herpes zoster of other locations. While the incidence of CVA 12 months following HZO was rare (1.6 per cent), the risk was highest immediately following HZO, with median time to CVA of 2.3 months. Interestingly, it was found that patients who received prompt antiviral therapy had a 76.2 per cent lower hazard of CVA.11
Current literature reports that the increased risk of CVA following HZO is most pronounced in individuals younger than age 40.4,11 Promoting prompt antiviral commencement and vaccination against herpes zoster is crucial in reducing its associated ocular and cerebrovascular burden. Vaccination has been observed to decrease the risk of CVA in older adults.
Prompt commencement of antiviral therapy is key in optimal management of HZO.5,9 The administration of an antiviral agent within 72 hours of rash onset has been shown to reduce acute HZO pain as well reduce the frequency and severity of ocular complications, including anterior uveitis.10,12
Valacyclovir 1000mg can be prescribed three times daily for 10 days. Another option is to prescribe acyclovir 800mg five times daily. Antiviral dosage may need to be renally adjusted, so it is ideal to monitor renal function.9
An age-related decline in immunity is thought to contribute to greater risk for herpes zoster among adults aged 50 and over. Vaccination offers the best protection from shingles and its associated complications.13,14
The Shingrix vaccine is a recombinant vaccine consisting of a varicella zoster virus glycoprotein and an adjuvant system that stimulates the immune response. The glycoprotein chosen is the most abundant viral surface glycoprotein in both varicella zoster virions and infected cells, and the adjuvant system comprises two immunostimulants.15
Two phase III trials evaluated the efficacy of Shingrix against herpes zoster in those aged 50 or older (ZOE-50 trial) and in those aged 70 or older (ZOE-70 trial). The overall vaccine efficacy was 97.2 per cent among those aged 50 or older, and 91.3 per cent among those aged 70 or older.16,17
Shingrix is the shingles vaccine used in New Zealand and requires two doses to be administered two to six months apart. The vaccine is approved and recommended for those aged 50 or older, and those aged 18 or older at increased risk of herpes zoster.
However, only adults aged 65 are eligible for a free first dose of Shingrix vaccine, and the first dose must be administered within 12 months of turning 65 to be eligible for a free second dose. From 1 July, it will also be funded for some immunocompromised people aged 18 or older. Shingrix is readily available in New Zealand but can cost $600–$800 for those who are not eligible for free vaccination.13,14
Vince Wilkinson is a fellow in ophthalmology at the University of Auckland. Rachael Niederer is an ophthalmologist and uveitis and medical retina specialist at Auckland Eye and Greenlane Clinical Centre, and a senior lecturer at the University of Auckland
You can use the Capture button below to record your time spent reading and your answers to the following learning reflection questions, which align with Te Whanake reflection requirements (answer three or more):
- What were the key learnings from this activity?
- How does what you learnt benefit you, or why do you appreciate the learning?
- If you apply your learning, what are the benefits or implications for others?
- Think of a situation where you could apply this learning. What would you do differently now?
- If an opportunity to apply this learning comes up in the future, what measures can be taken to ensure the learning is applied?
- Can you think of any different ways you could apply this learning?
- Are there any skills you need to develop to apply this learning effectively?
We're publishing this article as a FREE READ so it is FREE to read and EASY to share more widely. Please support us and our primary care education resources – subscribe here
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc 2009;84(3):274–80.
2. Motswaledi MH. Herpes zoster (shingles). S Afr Fam Pract 2018;60(4):28–30.
3. Ayoade F, Kumar S. Varicella-zoster virus (chickenpox). In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022.
4. Szeto SK, Chan TC, Wong RL, et al. Prevalence of ocular manifestations and visual outcomes in patients with herpes zoster ophthalmicus. Cornea 2017;36(3):338–42.
5. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008;115(2 Suppl):S3–12.
6. Kalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF. Eye and periocular skin involvement in herpes zoster infection. Med Hypothesis Discov Innov Ophthalmol 2015;4(4):142–56.
7. Lee HL, Yeo M, Choi GH, et al. Clinical characteristics of headache or facial pain prior to the development of acute herpes zoster of the head. Clin Neurol Neurosurg 2017;152:90–94.
8. Niederer RL, Meyer JJ, Liu K, Danesh-Meyer HV. Herpes zoster ophthalmicus clinical presentation and risk factors for loss of vision. Am J Ophthalmol 2021;226:83–89.
9. Thean JH, Hall AJ, Stawell RJ. Uveitis in herpes zoster ophthalmicus. Clin Exp Ophthalmol 2001;29(6):406–10.
10. Cobo M, Foulks GN, Liesegang T, et al. Observations on the natural history of herpes zoster ophthalmicus. Curr Eye Res 1987;6(1):195–99.
11. Meyer JJ, Liu K, Danesh-Meyer HV, Niederer RL. Prompt antiviral therapy is associated with lower risk of cerebrovascular accident following herpes zoster ophthalmicus. Am J Ophthalmol 2022;242:215–20.
12. Tugal-Tutkun I, Cimino L, Akova YA. Review for Disease of the Year: Varicella zoster virus-induced anterior uveitis. Ocul Immunol Inflamm 2018;26(2):171–77.
13. Maltz F, Fidler B. Shingrix: A new herpes zoster vaccine. P T 2019;44(7):406–33.
14. Te Whatu Ora. Shingles vaccine. May 2024.
15. Heineman TC, Cunningham A, Levin M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr Opin Immunol 2019;59:42–48.
16. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372(22):2087–96.
17. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016;375(11):1019–32.
Figure 2: Bakacs T. Healing of severe herpes zoster ophthalmicus within a few days: An autobiographical case report. Cureus 2021;13(12): e20303.









![New Zealand Doctor Rata Aotearoa editor Barbara Fountain, RNZCGP president elect and Tauranga-based specialist GP Luke Bradford, Ministry of Health clinical chief advisor rural health Helen MacGregor, and Health New Zealand Te Whatu Ora clinical director primary and community care Sarah Clarke [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/1.%20Barbara%20Fountain%2C%20Luke%20Bradford%2C%20Helen%20MacGregor%20and%20Sarah%20Clarke.jpg?itok=091NETXI)
![Ngāti Porou Oranga specialist GP Elina Pekansaari and Te Nikau Hospital specialist in general practice and rural hospital medicine David Short [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/2.%20Elina%20Pekansaari%20and%20David%20Short.jpg?itok=h5XfSBVM)
![Locum specialist GP Margriet Dijkstra and OmniHealth regional operations manager (southern) Patricia Morais-Ross [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/3.%20Margriet%20Dijkstra%20and%20Patricia%20Morais-Ross.jpg?itok=jkrtRfJC)
![Golden Bay dairy farmer and dairy industry health and safety doctoral student Deborah Rhodes, and Golden Bay Community Health specialist GP Rachael Cowie [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/4.%20Deborah%20Rhodes%20and%20Rachael%20Cowie.jpg?itok=oM0_GcJc)
![Hauora Taiwhenua clinical director rural health Jeremy Webber, Australian College of Rural and Remote Medicine president Rod Martin and Observa Care director of business operations Deborah Martin, the wife of Dr Martin [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/5.%20Jeremy%20Webber%2C%20Rod%20Martin%20and%20Deborah%20Martin%2C%20the%20wife%20of%20Dr%20Martin.jpg?itok=P_aGmX_H)
![Spark Health chief executive John Macaskill-Smith and client director Bryan Bunz [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/6.%20John%20Macaskill-Smith%20and%20Bryan%20Bunz.jpg?itok=5yJvVZ0I)
![Associate dean (rural) Kyle Eggleton, third-year medical student Roselle Winter, and second-year pharmacy student Alina Khanal, all from the University of Auckland [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/7.%20Kyle%20Eggleton%2C%20Roselle%20Winter%20and%20Alina%20Khanal.jpg?itok=RQLd3TEs)
![Health New Zealand Te Whatu Ora clinical editor and specialist in general practice and rural hospital medicine Anu Shinnamon, and Whakarongorau chief clinical officer Ruth Large [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/8.%20Anu%20Shinnamon%20and%20Ruth%20Large.jpg?itok=i5TMswY9)
![Te Kahu Hauora Practice specialist GP Jane Laver and Ngāti Kahungunu ki Tāmaki-nui-a-Rua chief operations manager Tania Chamberlain [Image: NZD]](/sites/default/files/styles/thumbnail_cropped_100/public/2025-05/9.%20Jane%20Laver%20and%20Tania%20Chamberlain.jpg?itok=jtMklaCZ)